In the battle against aggressive breast cancer, a once-elusive target is now within reach—thanks to a breakthrough from a team from the Faculty of Medicine at Hebrew University. Dr. Raphael Benhamou and M.Sc. student Liann Kassabri have developed innovative druglike molecules capable of degrading HuR, a key RNA-binding protein that stabilizes oncogenes and fuels cancer progression.
HuR (also known as ELAVL1) has long been labeled “undruggable” due to its structural flexibility and lack of a conventional active site. Overexpressed in many cancer types—particularly breast cancer—HuR fortifies malignant cells by protecting mRNAs that drive cell growth and survival.
“We knew that simply blocking HuR wasn’t enough,” says Dr. Benhamou. “We needed to eliminate it altogether.” Strikingly, this elimination led to a three to four orders of magnitude improvement in anticancer properties compared to traditional HuR-binding molecules that do not induce degradation.
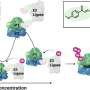
The research team turned to two cutting-edge therapeutic strategies: PROTACs (Proteolysis-Targeting Chimeras) and molecular glues. While both approaches harness the cell’s own protein-disposal machinery, molecular glues stand out for their small size, favorable pharmacokinetics, and oral bioavailability—traits prized in drug development.
After synthesizing and testing dozens of candidates, one compound in particular—MG-HuR2—emerged as a front-runner. “MG-HuR2 not only met every major druglikeness criterion, it demonstrated powerful activity at ultra-low concentrations,” says Kassabri.
In breast cancer cell lines, MG-HuR2 reduced HuR levels by up to 85%, disrupted the expression of downstream oncogenes like Bcl2 and FOXQ1, and significantly inhibited cell proliferation, survival, and 3D tumor spheroid growth. The research is published in the journal JACS Au.
A striking feature of these degraders is their biphasic “hook effect.” While activity drops off at intermediate doses—a known PROTAC quirk—the team discovered a rebound in efficacy at higher concentrations.
This unique behavior, not previously observed, was further investigated through computational modeling, which revealed that it stems from the compounds’ ability to engage two distinct RNA-binding pockets on HuR. This finding may open new avenues for targeting other dynamic RNA-binding proteins.
“The degradation pattern was unusual,” explains Dr. Benhamou. “But rather than a flaw, it turned out to be a clue. Our degraders bind at multiple sites, giving them a broader reach and potentially more durable effects.”
The implications are profound. Beyond breast cancer, HuR is implicated in various malignancies and inflammatory diseases. With MG-HuR2 and its peers, researchers now have a roadmap for developing drugs against a class of proteins previously thought beyond the reach of modern medicine.
For now, MG-HuR2 represents a promising lead compound and a milestone in targeted protein degradation. As Dr. Benhamou puts it, “We’re not just inhibiting cancer’s messages—we’re dismantling its messengers.”
More information:
Liann Kassabri et al, Druglike Molecular Degraders of the Oncogenic RNA-Binding Protein HuR, JACS Au (2025). DOI: 10.1021/jacsau.5c00551
Citation:
Targeting the ‘undruggable’: New molecular degraders offer hope for aggressive breast cancer (2025, July 23)
retrieved 23 July 2025
from https://medicalxpress.com/news/2025-07-undruggable-molecular-degraders-aggressive-breast.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.